Contained Tissue Extraction System
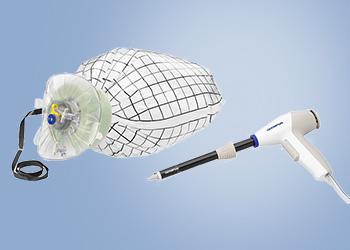
Tissue Management System
Contained Tissue Extraction System
OLYMPUS ANNOUNCES NEW FDA GUIDANCE AND LABELING CHANGES AFFECTING PNEUMOLINER, THE ONLY 510(K)-CLEARED CONTAINMENT DEVICE.
View Press Release
PNEUMOLINER FDA-REQUIRED SURGEON TRAINING:
Contained Tissue Extraction System

PneumoLiner
Containment Device

The PneumoLiner is uniquely designed to:
- Conform to each patient's abdominal size, avoiding intra-abdominal folding of material which can restrict movement, visualization, or inadvertent capture by the morcellator.
- Provide a barrier between target tissue and non-targeted abdominal contents.
- Maintain a barrier to the escape of fluids, cells and tissue fragments.
FDA Clearance & Letters:
WARNING: Information regarding the potential risks of a procedure with this device should be shared with patients. Uterine tissue may contain unsuspected cancer. The use of laparoscopic power morcellators during fibroid surgery may spread cancer. The use of this containment system has not been clinically demonstrated to reduce this risk.
CONTRAINDICATIONS: Do not use on tissue that is known or suspected to contain malignancy. Do not use for removal of uterine tissue containing suspected fibroids in patients who are: post-menopausal or over 50 years of age; or candidates for en bloc tissue removal, through the vagina or via a mini-laparotomy incision. Do not use in women with undiagnosed uterine bleeding. Do not use this device on patients with known or suspected allergies to polyurethane. Do not use where the abdominal wall thickness is larger than 10cm. This device should only be used by physicians who have completed the formal validated required training program administered by Olympus and/or Advanced Surgical Concepts.
For more information, please read the full PneumoLiner Instructions for Use for indications, additional contraindications, warnings and precautions.
For additional information, please view the videos below from AAGL 2015.
Guardenia™
The Guardenia™ is a new system that is designed to prevent the escape of cells into the abdominal cavity during extracorporeal manual morcellation. It's integrated guard provides protection against inadvertent damage from sharp surgical instruments around the incision.

- Guardenia allows 50% more space for the same linear incision size as offered by other marketed devices.
- Guardenia provides a larger operating space through the incision than the Alexis CES Guard as it encroaches less into the incision.1
- Guardenia is securely anchored in the incision and cannot pop out during tissue extraction.
- Guardenia provides complete protection of the Bag and the incision over a larger area across all incision sizes (25 mm - 60 mm) and abdomen thicknesses (10 mm - 80 mm).
1. Data on file as of 9/17/21
Supporting materials on file with manufacturer and distributor
Videos
- How to Handle the PneumoLiner Containment Device
-
Total Laparoscopic Hysterectomy and Contained Tissue Extraction with the Pneumoliner and PK Morcellator
- Tony Shibley, MD - Total Laparoscopic Hysterectomy with Contained Tissue Extraction with the PK Morcellator and PneumoLiner
- LESS Myomectomy with PK Needle and Contained Tissue Extraction with the PK Morcellator and PneumoLiner
- First Clinical Case: Prof. Marit Lieng, Dr. Karin Langeland, Dr. Thomas Thaulow - Oslo University Hospital
- In the Best Interest of Your Patients: How to Influence Hospital Policy on Contained Tissue Extraction
-
The Morcellation
Controversy
& Lessons Learned (Intro):
Trials, Tribulations, and
Alternative Solutions
- Tony Shibley, MD -
The Morcellation
Controversy
& Lessons Learned (Part 2):
The Tradeoffs of Abdominal
vs. Laparoscopic
Hysterectomy with
Morcellation
- Matthew Siedhoff, MD -
The Morcellation
Controversy
& Lessons Learned (Part 3):
The Quest for Safe Tissue
Extraction
- Tony Shibley, MD -
The Morcellation
Controversy
& Lessons Learned (Part 4):
Vaginal Hysterectomy as an
Alternative to Laparoscopy
- David B. Shwartz, MD


PATIENT EXPERIENCES
Katie's Story
When a hearing-impaired patient needs surgery, Kate, is the person to translate important information to them via sign language. She loves her work as a medical interpreter so, when uterine fibroids were limiting her ability to be at work, she felt a loss of purpose that her career gave to her.
Rachel's Story
“Most of us who work in the O.R. know that minimally invasive surgery is better for patients from a safety and recovery perspective,” Rachel said. “Minimally invasive approaches have been shown to result in shorter hospital stays, faster healing times, and cosmetic benefits associated with less significant scarring.”

Different Women, Different Needs.
At Olympus, we know it’s important that you feel at ease discussing health issues with your physician. Whether you are preparing for an initial visit or seeking more information when considering treatment options, we offer resources that will provide insight and help you in making an informed decision.
News
- The FDA recommends performing contained morcellation in women when laparoscopic power morcellation is appropriate
- The Wall Street Journal: Five Years After Cancer Warnings, Some Hospitals Still Use Hysterectomy Tool
- Contemporary OB/GYN: FDA Report a Disservice to Fibroids Patients
- VIDEO: Tips for performing contained power morcellation
-Tony Shibley, MD - Newly available tissue containment system brings back power morcellation to advanced MIG surgeons
– Lila O’Connor, Editor - Tissue extraction: Can the pendulum change direction?
– Arnold P. Advincula, MD - A containment system for morcellation
– Jubilee Brown, MD
Training
Events
Society of Endometriosis and Uterine Disorders (SEUD) 2019
May 16th – 18th,
Montreal, CanadaSociety of Laparoendoscopic Surgeons (SLS) 2019
September 4th – 7th,
New Orleans, LAAmerican College of Obstetricians (ACOG) Armed Forces District Meeting 2019
September 7th – 11th,
San Diego, CAAmerican Urogynecologic Society (AUGS) 2019
September 24th – 28th,
Nashville, TNAmerican Society for Reproductive Medicine (ASRM) 2019
October 12th – 16th,
Philadelphia, PAAmerican Society for Health Care Risk Management (ASHRM) 2019
October 13th – 16th,
Baltimore, MDAmerican Association of Gynecologic Laparoscopists (AAGL) 2019
November 9th – 13th,
Vancouver, B.C., CanadaPelvic Anatomy & Gynecologic Surgery Symposium (PAGS) 2019
December 12th – 14th,
Las Vegas, NVSociety of Gynecologic Oncology (SGO) 2020
March 28th – 31st,
Toronto, CanadaThe Association of periOperative Registered Nurses (AORN) 2020
March 28th – April 1st,
Anaheim, CASociety of Gynecologic Surgeons (SGS) 2020
March 29th – April 1st,
Jacksonville, FLAmerican College of Obstetricians (ACOG) 2020
April 24th – 27th,
Seattle, WA
Additional Reading

- What a Black Box Warning Can Do for Innovation and Patient Safety – K. Anthony Shibley - PSQH. 2018 October
- Practice Patterns and Complications of Benign Hysterectomy Following the FDA Statement Warning Against the Use of Power Morcellation – Multinu et al. - JAMA Surg. 2018 Jun 20; 153(6): e180141
- Morcellation during Uterine Tissue Extraction: An Update – AAGL - J Minim Invasive Gynecol. 2018 May-Jun; 25(4): 543-550
- A Medical-Legal Review of Power Morcellation in the Face of the Recent FDA Warning and Litigation – Ton et al. - J Minim Invasive Gynecol. 2015 May-Jun; 22(4): 564-72
- Tissue Extraction Techniques During Laparoscopic Uterine Surgery – Cohen et al. - J Minim Invasive Gynecol. 2018 Feb; 25(2): 251-256
- Choosing the Route of Hysterectomy for Benign Disease - Matteson, KA et al- American College of Obstetrics and Gynecologists. June 2017
- The Unintended Consequences of an FDA Warning: The Case of Power Morcellation in Myoma Surgery. – Po, L and Lee, PE - J Minim Invasive Gynecol. 2016;23:597-602
- An Open Letter to the Food and Drug Administration Regarding the Use of Morcellation Procedures in Women Having Surgery for Presumed Uterine Myomas. – Parker, W et al. - J Minim Invasive Gynecol. 2016 Mar-Apr; 23(3):303-308
- Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. – Harris, J et al. - Am J Obstet Gynecol. 2016 Jan; 214(1):98.e1-98.e13
- A Medical-Legal Review of Power Morcellation in the Face of the Recent FDA Warning and Litigation – Ton et al. - J Minim Invasive Gynecol. 2015 May-Jun; 22(4): 564-72
- Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. – Siedhoff, MT et al. - Am J Obstet Gynecol. 2015 May;212(5):591.e1
- Outcome of Occult Uterine Leiomyosarcoma After Surgery for Presumed Fibroids: A Systematic Review. – Pritts, et al. - 2015 Jan. J Minim Invasive Gynecol. 22(1):26–33